Home → Research about SCI/D → Printer Friendly Version
Research about SCI/D
- 1. Studies Currently Recruiting People with SCI/D or SCI Clinicians
- 1.1. United Spinal Disclaimer
- 1.2. Scale up Trial of Project WOWii to Increase Exercise Among People with Spinal Cord Injury
- 1.3. Build with us the next generation of hands-free walking exoskeleton
- 1.4. Identity-First Language or Person-First Language
- 1.5. Research Study on the Experiences of Social Media and Connectedness
- 1.6. Standardizing a Core Set of Patient Reported Outcomes for Spinal Cord Injury Care-McGill University
- 2. Functional Electrical Stimulation (FES)
- 3. Understanding SCI research
- 4. United Spinal Association Research Related Webinars
- 5. General research information
- 6. Specific research information
- 6.1. BrainGate Neural Interface System
- 6.2. Lower Extremity Functional Electrical Stimulation Cycling study
- 6.3. Regeneration & Repairing Adult Stem Cells
- 6.4. Review of SCI Human Trials
- 6.5. Spinal Cord Implant Allows Paraplegics to Walk Again, Scientists Say-February 2022
- 6.6. UNC - Spinal Motor Neurons
- 6.7. Women with disabilities
- 7. Learn about Participation in SCI Research
- 7.1. Experimental Treatments for Spinal Cord Injuries: What you should know if you are considering participation in a clinical trial.
- 7.2. How do I learn if a therapy is valid and safe
- 7.3. Stem Cell Treatments: What to Ask
- 7.4. 60 Minutes expose on stem cell program fraud
- 7.5. Video: Geron Stem Cell Clinical Trial Participant Shares Her Experience
- 8. Where do I find ongoing research studies?
- 8.1. Institute for Functional Restoration
- 8.2. SCITrials.org
- 8.3. SCITrialsFinder.net
- 8.4. ClinicalTrials.gov
- 8.5. California Institute for Regenerative Medicine
- 8.6. SCIRE: SCI Research Evidence
- 8.7. UAB Spinal Cord Injury Model System Information Network
- 8.8. Miami Project to Cure Paralysis
- 8.9. Unite 2 Fight Paralysis
- 9. Will my insurance reimburse for participating in SCI research?
- 10. Websites to learn about SCI research
- 11. Articles and Videos about SCI research
- 11.1. Spinal Cord Injury Research and the Hope for Cure: Where are we Today? (2013)
- 11.2. Spinal Cord Injury Research Evidence (SCIRE)
- 11.3. CareCure Forums - Article Abstracts
- 11.4. Hope Through Research (NINDS)
- 11.5. Research: Stem Cells
- 11.6. Stem Cell Therapies
- 11.7. Survive, Subsist, Succeed: Spinal Cord Injury
- 12. Advocacy Groups
- 13. SCI Statistical sources
- 14. Advance Directives (donating your spinal cord for scientific research)
1. Studies Currently Recruiting People with SCI/D or SCI Clinicians
1.1. United Spinal Disclaimer
United Spinal Association provides information on spinal cord injury/disorders research as a service to its members. United Spinal Association does not specifically endorse any research study and this information is provided for your convenience only.
1.2. Scale up Trial of Project WOWii to Increase Exercise Among People with Spinal Cord Injury
Scale up Trial of Project WOWii to Increase Exercise Among People with Spinal Cord Injury
Getting into a consistent fitness routine and staying motivated can be hard. If you are someone with a spinal cord injury (SCI) who agrees with that statement, then we encourage you to join the Project Workout on Wheels Internet Intervention (WOWii)! With Project WOWii, we aim to help you incorporate exercise into your lifestyle over 4 months in a fun, approachable way using an online format. Eligible participants will meet on Zoom weekly and work through online modules, collaborate with fellow participants and motivate each other to start and stick with an accessible and individualized exercise program. Participants will get to keep the provided exercise starter pack and exercise tracking watch after study participation.
To participate, you must:
- Be 18 years of age or older.
- Have an SCI.
- Be able to perform arm-based exercise.
- Have internet access.
Sound interesting? If you are ready to sign up or still have a few questions, don’t hesitate to contact us at wowiiproject@gmail.com or 720-828-3989 today!
1.3. Build with us the next generation of hands-free walking exoskeleton
Do you picture yourself using an autonomous, self-stabilized, hands-free exoskeleton in your daily life? Help build the future generation of exoskeletons! Wandercraft is a French company that has developed the first self-balanced lower limbs exoskeleton. What is the perfect exoskeleton for you? Let us know by answering the following survey. Participation takes less than 10 minutes.
https://bit.ly/walking-exo-survey
Wandercraft a leading healthtech company in dynamic robotics and exoskeleton technology that developed the first self-balanced lower limbs exoskeleton: Atalante. It enables walking impaired individuals to walk autonomously.
Wandercraft is developing a personal version of the exoskeleton for outdoor and home use. It will enable people with reduced mobility to regain autonomy in their daily activities and improve their health, both at home and elsewhere.
In order for this exoskeleton to be really useful to a lot of people, we have launched a study among people with reduced mobility.
Wandercraft, pioneer of walking robotics
About Wandercraft
Wandercraft was founded in 2012. A group of engineers refused to accept the status quo of wheelchair use, believing they could create a better solution for mobility and autonomy. Wandercraft builds autonomous walking exoskeletons. Its first version, Atalante, was commercialized in 2019 and is used by rehabilitation and neurological hospitals in Europe and North America. Atalante provides innovative care for many patients based on realistic, hands-free, crutch-free locomotion.
Wandercraft is developing a personal version of the exoskeleton for outdoor and home use. It will enable people with reduced mobility to regain autonomy in their daily activities and improve their health, both at home and elsewhere.
For more information: www.wandercraft.eu
1.4. Identity-First Language or Person-First Language
A team of researchers at the University of Washington is conducting research to gather evidence on the preference between identity-first & person-first language by & for disabled people. Please consider taking part in a survey to state your preference: https://bit.ly/disability-language-survey. Survey should only take about 5 mins. Please share.
1.5. Research Study on the Experiences of Social Media and Connectedness
1.6. Standardizing a Core Set of Patient Reported Outcomes for Spinal Cord Injury Care-McGill University
McGill University is looking for volunteers to take part in a study to identify & agree upon a minimum set of patient reported core domains that assess quality of life for SCI.
If
you are someone with SCI, caregiver, health care professional, decision maker
or researcher having experience with SCI, please consider helping by
completing the survey:
English: https://surveys.mcgill.ca/ls/895759?lang=en
French: https://surveys.mcgill.ca/ls/895759?lang=fr
See attached flyer
2. Functional Electrical Stimulation (FES)
2.1. FES Equipment-Myolyn
Myolyn's Myocycle/Get The Facts
Contact page & Phone
After a spinal cord injury, many people find themselves needing help for a lot of basic daily activities. Even those who can do most things independently still miss the mobility and spontaneity they used to have, and many start to worry about the long-term health consequences of sitting in a wheelchair.
We all hope for a cure. But while we’re waiting for a cure that still seems elusive, real people need real help today.
What if we could restore the lost functions of paralysis, one by one? Hand function. Sexual function. Bladder function. Trunk stability. Standing. Stepping. Technologies exist that have the potential to do this; they’re called neuromodulation systems. We have a third generation design that rests on more than 30 years of research, and we are ready to start studying it in people so we can bring it to market.
2.2. FES Equipment-Restorative Therapies
Restorative Therapies — iFES Systems for Rehabilitation at Clinics and at Home
Contact Form
At Restorative Therapies, we offer industry-leading iFES systems for people with neurological impairments across the full continuum of care. Our iFES systems are evidence of our unwavering commitment to innovation backed by years of sound clinical research.
Since 2005 Restorative Therapies systems have been cleared by the FDA to:
- Reduce muscle atrophy
- Reduce muscle spasms
- Improve local circulation
- Maintain or increase range of motion
- Facilitate muscle re-education
Our iFES systems are physician-prescribed, FDA Class II medical devices.
Please see Attached Referal form, Factsheet Patient handout
2.3. FES Promotes Recovery in Chronic SCI
FES article in Science Daily
4 March 2013
Functional Electrical Stimulation Cycling Promotes Recovery in Chronic Spinal Cord Injury
A new study by Kennedy Krieger Institute's International Center for Spinal Cord Injury (Epub ahead of print) finds that long-term lower extremity functional electrical stimulation (FES) cycling, as part of a rehabilitation regimen, is associated with substantial improvements in individuals with chronic spinal cord injury (SCI). Improvements include neurological and functional gains, as well as enhanced physical health demonstrated by decreased fat, increased muscle mass and improved lipid profile.
2.4. Transcutaneous Stimulation
NeuroRecoveryTechnologies group reports success in returning voluntary motor function in five men living with chronic, complete SCI through non-invasive transcutaneous stimulation.
The company is focused on the development of two unique spinal cord neuromodulator systems (electric stimulation devices) sharing platform technology to address a significant unmet clinical need. The first is a non-invasive external system, classified by the FDA as a Class II De Novo 510k, for those patients with partial to complete paralysis. The second system is implantable, a PMA Class III device, used to optimally treat individuals with severe incomplete & complete paralysis. Each system is composed of complementary electrodes plus a wireless pulse generator containing proprietary circuitry and algorithms with our discovered formula for reactivating neural circuits. The systems facilitate customized programming and independent control of multiple stimulation sites.
3. Understanding SCI research
3.1. SCI2020-Decade of Disruption in Research-Event Video Archived
SCI 2020 Videos Now Available
The National Institutes of Health (NIH) has posted videos from
the conference SCI 2020: Launching a Decade for Disruption in Spinal Cord
Injury Research. The February 2019 conference featured presentations and
panel discussions on current and future opportunities for research and
development in SCI. Members of the NIDILRR-grantee community participated in
the conference, including a keynote presentation from Michael Boninger, MD,
principal investigator for the University of Pittsburgh Model Center on SCI. In
addition to the videos, NIH has posted poster session abstracts and reference
lists for the keynote presentations.
3.2. Understanding Research: Those Scary Statistics
Understanding Research: Those Scary Statistics is an educational brochure developed by Craig Hospital for people with spinal cord injury. Revised 2015.
3.3. Understanding Research: Medical & Research Articles
Ever have trouble making sense out of articles in medical, scientific, and research magazines? This brochure from Craig Hospital will give you some pointers as you try to wade through all the techno-jargon you find. Revised 2015.
3.4. The Controversy Over Walking & Research
SCI Forum presentation "To Walk or Roll: The Controversy over Walking and Research". Presented on May 17, 2012 at the University of Washington Medical Center by the Northwest Regional SCI System, Department of Rehabilitation Medicine, University of Washington.
Will I ever walk again?" This is frequently the first question a patient with a new spinal cord injury asks. A focus on walking is normal after what can be a devastating injury. However, over time the focus on walking often fades and other issues that impact daily living move to the top of the wish list. Is there peril if individuals with SCI and researchers focus on walking too much? Where should we be putting our scant SCI research dollars? Our guest speaker, Michael Boninger, MD, examines this complex issue from the standpoint of both a dedicated physiatrist who has worked with SCI patients for over 20 years and a prominent researcher seeking solutions to the many problems caused by these injuries. Dr. Boninger is professor and chair of the Physical Medicine and Rehabilitation Department at the University of Pittsburgh, where he also serves as the medical director of the Human Engineering Research Laboratory and director of the University of Pittsburgh Model Center on Spinal Cord Injury.
4. United Spinal Association Research Related Webinars
4.1. All You Need to Know About Direct Nerve Stimulation 7/20/16
All You Need to Know About Direct Nerve Stimulation 7/20/16
This webinar introduces the latest technology applications for direct nerve stimulation for both the peripheral and central nervous systems and the published research supporting or negating them. Take away what you need to know to make an informed decision.
View Webinar Video | View Webinar PDF | Webinar Transcript
4.2. "Becoming a Lab Rat" Lessons learned from participating in a Clinical Trial- 09/25/13
"Becoming a Lab Rat" Lessons learned from participating in a Clinical Trial 09/25/13
This webinar provides resources to learn about available clinical trials, how to know if it is a valid trial, and what to expect by being a clinical trial participant. Presented by:
Kim Anderson-Erisman, PhD, Director of Education, Miami Project to Cure Paralysis
Jennifer French, MBA, Executive Director, Neurotech NetworkUSAWebinar_ClinicalTrial_Sept2013Final2.ppt
4.3. Breakthroughs in Stem Cell Therapies 10/23/14
Breakthroughs in Stem Cell Therapies: 10/23/14
This United Spinal Association webinar discusses the differences in stem cells, the status of research and what you need to know before considering treatment.
Presented by: Jennifer French, Executive Director; Neurotech Network and Kim Anderson-Erisman, Ph.D.; Research Associate Professor, Department of Neurological Surgery-Director of Education, The Miami Project to Cure Paralysis; University of Miami Miller School of Medicine.
5. General research information
5.1. Model Systems Knowledge Translation Center
The Model Systems Knowledge Translation Center (MSKTC) summarizes research, identifies health information needs and develops systems for sharing information for the National Institute on Disability, Independent Living, & Rehabilitation Research (NIDILRR) model systems programs in traumatic brain injury, spinal cord injury and burn injury.
5.2. National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR)
National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR)
NIDILRR is a federal government grants-making agency that sponsors grantees to generate new disability and rehabilitation knowledge and promote its use and adoption.
5.3. NIH Clinical Research Trials and You
NIH has created a new website, NIH Clinical Research Trials and You (clinicalresearchtrials.nih.gov) to help people learn more about clinical trials, why they matter and how to participate.
Research has shown that some of the greatest challenges to recruitment of volunteers are the lack of general knowledge about what trials involve, where they are carried out, and who may participate. The new, centralized resource will make it easier for the public and health professionals to learn about clinical trials and how people can participate.
Visitors to the site will find information about:
. the basics of clinical trial participation
. experiences from clinical trial volunteers and explanations from researchers
. links on how to search for a trial or enroll in a research matching program
In addition, health care professionals can read about evidence-based strategies for talking with patients about trials, and find audience-tested posters to help promote trials in clinics and offices
6. Specific research information
6.1. BrainGate Neural Interface System
BrainGate is a brain implant system developed by the bio-tech company Cyberkinetics in 2003 in conjunction with the Department of Neuroscience at Brown University. A computer chip, which is implanted into the brain, monitors brain activity in the patient and converts the intention of the user into computer commands.
This brief newsclip shows the brain computer interface with implanted electrodes and a trial of its use with a quadriplegic man.
6.2. Lower Extremity Functional Electrical Stimulation Cycling study
A study published in the Journal of Spinal Cord Medicine by John W. McDonald, M.D., Ph.D., the neurologist who treated the late Christopher Reeve, finds that long-term lower extremity functional electrical stimulation (FES) cycling is associated with substantial improvements in individuals with chronic spinal cord injury. Prior to this study from Kennedy Krieger Institute's International Center for Spinal Cord Injury, the benefits of activity-based restorative therapy (ABRT) programs, such as FES cycling, were largely anecdotal despite being highly publicized in conjunction with the late-onset recovery demonstrated by actor and activist Christopher Reeve.
Some highlights of the study's findings include:
- · Improved motor function was observed in 80 percent of the FES group, compared to only 45 percent of control subjects.
- · Response in pinprick sensation was observed in 56 percent of the FES group compared with 25 percent of the control group.
- · 14 of the 25 FES subjects showed response in light touch scores compared to six of the 20 controls.
What is FES Cycling?
In FES cycling, small electrical pulses are applied to the paralyzed muscles of an individual, stimulating the muscles to cycle on a stationary recumbent bicycle. The repetitive activity simulates movement and offers cardiovascular exercise similar to that which an able-bodied individual achieves through walking, but this new research shows that the results go far beyond basic health benefits.
6.3. Regeneration & Repairing Adult Stem Cells
Stem Cells and Regeneration of the Spinal Cord: Practical Barriers and New Cell Technologies is an SCI Forum presentation of the Northwest Regional SCI System, Department of Rehabilitation Medicine, University of Washington. The forum was presented by:
Philip J. Horner, PhD
Associate Professor, Department of Neurological Surgery and Institute for Stem Cell and Regenerative Medicine, University of Washington.
6.4. Review of SCI Human Trials
This SCI Forum was presented on March 13, 2007, by the Northwest Regional SCI System, Department of Rehabilitation Medicine, University of Washington.
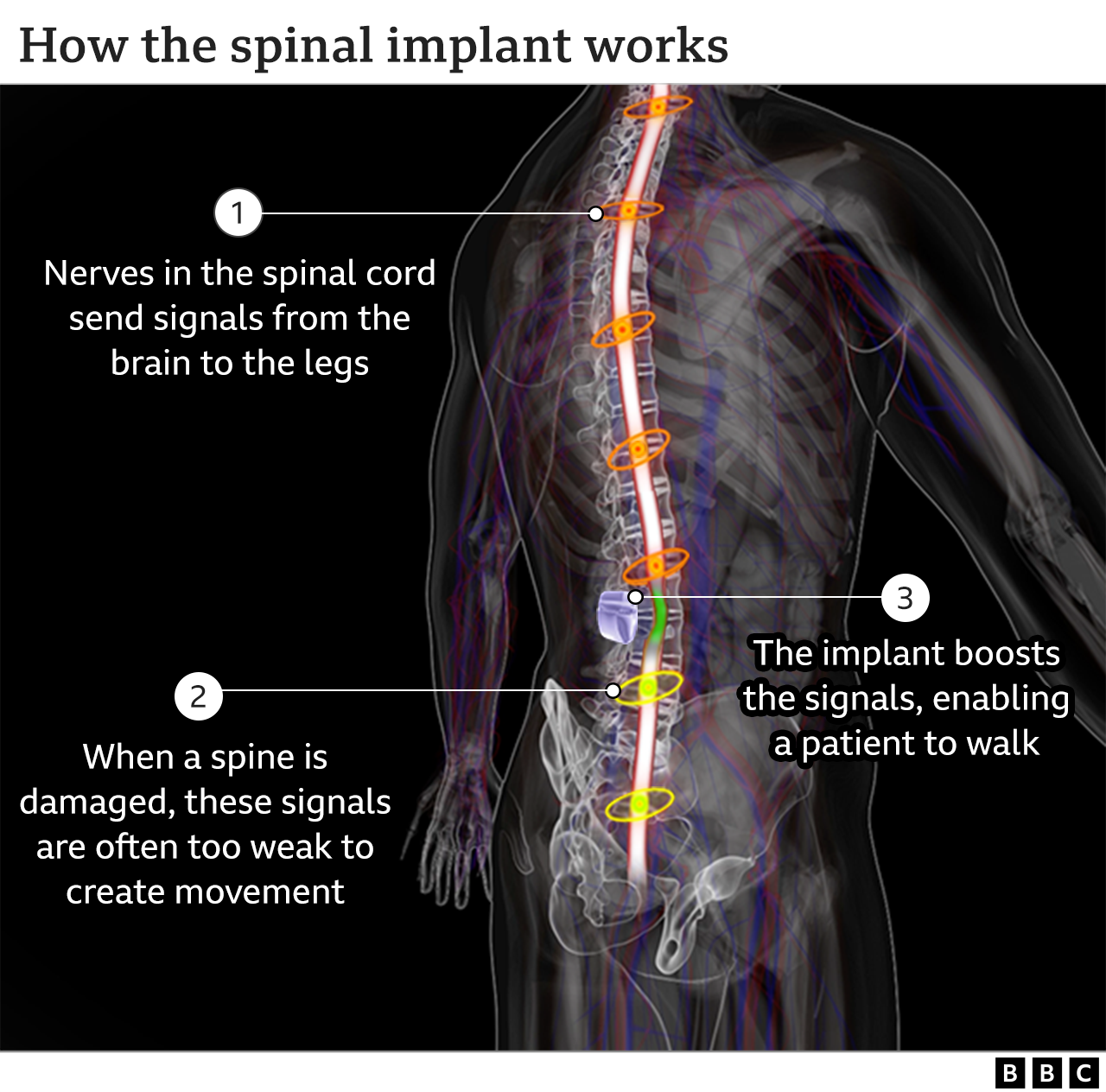
6.5. Spinal Cord Implant Allows Paraplegics to Walk Again, Scientists Say-February 2022
Via Web MD- Spinal Cord Implant Allows Paraplegics to Walk Again, Scientists Say
Feb. 7, 2022
Three men paralyzed with severe spinal cord injuries were able to walk again days after receiving a spinal cord implant that stimulates trunk and leg muscles -- a development scientists think could have broad application as a commercial product.
Scientists implanted 16-electrode devices into the epidural space on the men’s spines, between the vertebrae and the spinal cord membrane, CNN reported. The electrodes receive electrical currents from pacemakers implanted under the skin of their abdomens that are wirelessly controlled with a tablet computer, CNN said.
Michel Roccati of Italy, who lost his ability to walk in a 2017 motorcycle accident, said now that he has the implant, he can move around town with a walker and stand to take a shower.
"I am free," Roccati said. "I can walk wherever I want to."
The study was led by Jocelyne Bloch from Lausanne University Hospital and Grégoire Courtine of the Swiss Federal Institute of Technology. The results were published Monday in the journal Nature Medicine.
Electrical stimulation of the spine has been studied for years but hasn’t shown such immediate results.
In 2018, for example, the Mayo Clinic in the United States said a man paralyzed in a snowmobile accident was able to walk again with a spinal implant, but only after 22 weeks of physical therapy.
The men in the recent study had lost all voluntary movement below the site of their injuries but were able to take steps on a treadmill the day after surgery, CNN said.
"It's a very emotional moment, because [patients] realize they can step," Bloch said.
Physical therapy and three to four months of training was required before the men the Swiss study could complete actions such as climbing stairs or walking 500 meters independently, CNN said.
"For the first time, we have not only immediate effect -- though training is still important -- but also individuals with no sensation, no movement whatsoever, have been able to regain full standing and walking independently of the laboratory," Courtine said.
USA Today reported that the Swiss team hopes to begin a 50-100 patient clinical trial within a few years and eventually a 1,000-person trial to gain approval from the U.S. Food and Drug Administration. If approved, the technology could provide new hope for thousands of paralyzed people.
Courtine told USA Today the research team’s next goal is to control the electrodes with a cell phone.
The researchers said the FDA approved a "breakthrough devices" designation for the technology, which would allow people to obtain coverage through the Medicare Coverage of Innovative Technology program, CNN said.
Show Sources
Via BBC-Paralyzed Man Walks with Implant
A paralysed man with a severed spinal cord has been able to walk again, thanks to an implant developed by a team of Swiss researchers.
It is the first time someone who has had a complete cut to their spinal cord has been able to walk freely.
The same technology has improved the health of another paralysed patient to the extent that he has been able to become a father.
The research has been published in the journal Nature Medicine.
Michel Roccati was paralysed after a motorbike accident five years ago. His spinal cord was completely severed - and he has no feeling at all in his legs.
But he can now walk - because of an electrical implant that has been surgically attached to his spine.
Someone this injured has never been able to walk like this before.
The researchers stress that it isn't a cure for spinal injury and that the technology is still too complicated to be used in everyday life, but hail it nonetheless as a major step to improving quality of life.
I met Michel at the lab where the implant was created. He told me that the technology "is a gift to me".
"I stand up, walk where I want to, I can walk the stairs - it's almost a normal life."
It was not the technology alone that drove Michel's recovery. The young Italian has a steely resolve. He told me that from the moment of his accident, he was determined to make as much progress as he could.
"I used to box, run and do fitness training in the gym. But after the accident, I could not do the things that I loved to do, but I did not let my mood go down. I never stopped my rehabilitation. I wanted to solve this problem."
The speed of Michel's recovery amazed the neurosurgeon who inserted the implant and expertly attached electrodes to individual nerve fibres, Prof Jocelyne Bloch at Lausanne University Hospital
"I was extremely surprised," she told me. "Michel is absolutely incredible. He should be able to use this technology to progress and be better and better."
The research has been backed by Dr Ram Hariharan, a consultant at the Northern General Hospital in Sheffield who is independent of the research team and also speaks for the Spinal Injuries Association.
"They have done something that has not been done before.
"I have not heard of any study where they have put in an implant [into a patient with a complete spinal cord cut] and demonstrated muscle movements and improving balance, enough to stand and walk."
But he added more clinical trials needed to be carried out before he could be convinced that it was an effective treatment.
"We need more numbers [of patients] to show that it is first safe and that it significantly enhances their lives. Only then can it be taken forward."
Nerves in the spinal cord send signals from the brain to the legs. Some people are paralysed when the nerves are damaged through injury.
In Michel's case there's no signal at all to his legs because the spinal cord is completely severed. But the implant sends signals directly to his legs enabling him to walk, but only when the implant is on.
So far nine people have received the implant and regained the ability to walk. None of them use it to help them walk in their everyday lives - because it's too complicated at this stage. Instead, they use it to practise walking - which exercises their muscles, improves their health - and often, restores a little bit of movement.
David M'zee was one of the first patients to receive the implant. Like Michel, he was able to walk with the implant while using a walker. David's health improved to such an extent he has was able to have a baby girl with his partner Janine, something that was not possible after his accident in 2010.
His daughter, Zoe, is now one year old. When I was with them, she raced with her Dad with her baby walker and took great delight in beating him.
"It's really beautiful!" he beamed with fatherly pride.
"It is great fun. It's the first time I have been walking with her in that way - she with her baby walker, I with my walker."
Having a family has given David a huge amount of joy. And the implant has helped him in subtle, but important ways.
"It helps with the hypotension. I had it for so long. At first I didn't realise I had it. I was getting so tired from time to time.
"Once we found out that the implant can increase the blood pressure it was like 'Wow, that's how life can be!'"
"It's these small things that make a big difference," he told me.
There is still a long way to go before the technology can be used routinely to help paralysed people to walk, according to Prof Grégoire Courtine, who led the team that developed the technology at the École Polytechnique Fédérale de Lausanne (EPFL).
"This is not a cure for spinal cord injury. But it is a critical step to improve people's quality of life. We are going to empower people. We are going to give them the ability to stand, to take some steps. It is not enough, but it is a significant improvement."
A cure would require regeneration of the spinal cord, possibly with stem cell therapies, which are still at a very early stage of research. Prof Courtine believes that his implant technology could be used in conjunction with nerve regeneration treatments once they are ready.
Follow Pallab on Twitter
6.6. UNC - Spinal Motor Neurons
A Summary of Research in the Laboratory of David L. McIlwain, University of North Carolina School of Medicine
6.7. Women with disabilities
The mission of the Center for Research on Women with Disabilities is to promote, develop, and disseminate information to improve the health and expand the life choices of women with disabilities.
7. Learn about Participation in SCI Research
7.1. Experimental Treatments for Spinal Cord Injuries: What you should know if you are considering participation in a clinical trial.
Click here to download the easy-to-read, version 3 of the booklet, available through ICORD (International Collaboration on Repair Discoveries), for people with spinal cord injury, their families, friends and caregivers. You can also visit the following link to Download Questions to Ask / Summary.
7.2. How do I learn if a therapy is valid and safe
For any therapy or treatment you are considering, check to see if it is safe:
- Look for any peer reviewed published papers on the treatment. Peer review is important. This means that the scientist's/physician's work has been reviewed by peers (one with equal or some knowledge) in the field and has been approved for publishing. These include 'reviewed papers' from around the world.
- There should be background studies, pilot studies or pre-clinical studies published. The best compilation of medical studies is maintained at The National Institutes of Health, Library of Medicine. The link to the free library access is: http://www.ncbi.nlm.nih.gov/pubmed
- 'Reviewed' papers will be available even if the treatment may be 'brand new' or 'novel'
'Scam' or non-validated therapies should raise a Red Flag of caution.
- If you are considering such a therapy or treatment, you should request copies of published previous work AND references of the publishing (ie. Journal, date, publication) which is typically called 'pre-clinical' or pilot studies.
Many of the 'scam' treatments have not been published or reviewed
7.3. Stem Cell Treatments: What to Ask
The International Society of Stem Cell Research provides information for people considering stem cell treatment at the website, "A closer look at stem cell treatments" including, Stem Cell Treatments: What to Ask. There is certain information you should look into if you are considering a stem cell treatment, including a detailed description of the treatment and the science that supports it, the expected outcome and the risks.
7.4. 60 Minutes expose on stem cell program fraud
This a cautionary tale of a fraudlent stem cell program featured on CBS-60 Minutes where unrealistic improvements are promised and large sums required for un-proven stem cell treatments (aired 2/27/11).
7.5. Video: Geron Stem Cell Clinical Trial Participant Shares Her Experience
Katie Sharify is one of five people with spinal cord injuries to participate in the world's first clinical trial testing human embryonic stem cells. On November 14th, she spoke about her clinical trial experience with a group of scientists who were meeting to discuss the best ways of advancing stem cell clinical trials. CIRM communications manager Amy Adams interviewed Sharify and her doctor, Stephen McKenna.
8. Where do I find ongoing research studies?
8.1. Institute for Functional Restoration
The Institute for Functional Restoration or IFR, was created at Case Western Reserve University (CWRU) to make available interventions developed on campus that address the functions lost due to spinal cord injury or other paralytic conditions. Much different than a research organization, the IFR chooses programs that have been shown to be viable solutions for patients and then navigates them through the pathway to availability outside of a research trial. The Institute is funded by a combination of grants, philanthrophy and reimbursement.
8.2. SCITrials.org
SCITRIALS.ORG
is a new research trials site, which was created for and by the spinal cord injury community to connect researchers and people living with SCI.
It was created to address some of the challenges users were experiencing on existing SCI trial sites, by providing information on trials specific to SCI. Trials are filtered to contain studies from legitimate universities, research centers, and hospitals. Additionally, the site allows the user to save a search unique to their preferences and then receive email updates when relevant trials become available in their area.
To access the continually updated information in the site, simply sign up to use the free platform at SCITRIALS.ORG. We encourage site users to spread the news about this new tool throughout their networks.
SCITRIALS.ORG is a joint initiative of the North American Spinal Cord Injury Consortium (NASCIC) and www.endParalysis.org – with development provided by fuelService.org
8.3. SCITrialsFinder.net
The goal of the SCITrialsFinder.net website is to allow individuals with spinal cord injury (SCI), their families and health care professionals to get common language information about clinical trials as developed by experienced clinical investigators (called curations or curated trials). SCITrialsFinder.net has started curating trials looking for participants for studies of interventions targeting improvement of neurological and related functional outcomes, currently underway in North America, Europe and Australia. In addition to the curated trials, users can also read about all SCI related trials from clinicaltrials.gov.
8.4. ClinicalTrials.gov
ClinicalTrials.gov is a registry of federally and privately supported clinical trials conducted in the United States and around the world. ClinicalTrials.gov gives you information about a trial's purpose, who may participate, locations, and phone numbers for more details. This information should be used in conjunction with advice from health care professionals.
8.5. California Institute for Regenerative Medicine
California Institute for Regenerative Medicine (CIRM)
CIRM, California's Stem Cell Agency, was created by the voters of California in 2004 when they passed Proposition 71, which authorized $3 billion in funding for stem cell research in California. The agency funds stem cell research at institutions and companies throughout California with the goal of developing new therapies for deadly diseases and disorders.
8.6. SCIRE: SCI Research Evidence
Spinal Cord Injury Research Evidence (SCIRE): The SCIRE Project is a Canadian collaboration between scientists, clinicians and consumers. SCIRE covers a comprehensive set of topics relevant to SCI, including research in SCI in both the acute and rehabilitation stages. They have also created some resources for consumers with SCI.
8.7. UAB Spinal Cord Injury Model System Information Network
The University of Alabama at Birmingham Spinal Cord Injury Model System Center (UAB-SCIMS) provides links to research information as a community service to researchers and individuals with SCI. The UAB-SCIMS does not necessarily endorse or support any of the projects that are listed.
8.8. Miami Project to Cure Paralysis
Check out the Miami Project to Cure Paralysis
What Are Clinical Trials?
Clinical research is designed to answer specific questions about dysfunction and its treatment in humans. These questions may concern the cause of a medical or psychological condition or study the effectiveness and safety of a novel treatment or device.
Clinical research involves people who voluntarily give informed consent to serve as research participants without whom the research simply could not be done. Though volunteering for research does not guarantee a benefit, people who take part in clinical trials and studies make important contributions to the knowledge of, and progress toward, new treatments for Spinal Cord Injury (SCI) and its related problems.
A clinical trial is a carefully organized and controlled research study designed to test the safety and effectiveness of a novel treatment or device used for the first time for a particular medical or psychological condition.
The Miami Project conducts basic science research (laboratory setting) with the goal of making new discoveries and then translating them into successful clinical treatments for SCI through their Clinical Trials Initiative.
The Miami Project also carries out many clinical studies that address various problems associated with SCI, such as pain, spasticity, fatigue, fertility, fitness, rehabilitation, and other areas of concern for people with SCI. For more information, see "Current Studies". Through this research, scientists strive to discover valuable information about SCI that helps in development of new treatments.
How are Clinical Trials Introduced?
Scientists may invest years in basic science research, first in discovery and design of a new treatment and then in testing through experiments that model human disease. Once investigators complete "proof of concept" experiments, which establish a scientifically sound understanding of how a treatment works and potential benefit, the preclinical phase of research can begin.
The preclinical phase involves further research into the treatment's benefit through experimental models but also evaluates whether the treatment has the potential to cause harm. Investigators explore questions about possible damaging effects, such as the formation of tumors or toxicity from higher doses of a drug. These safety studies are very important to achieve approval to begin testing a new treatment in a human clinical trial.
Approval to perform human research at a given institution is received from an Institutional Review Board (IRB). An IRB is comprised of faculty, staff, and community members who are charged with ensuring the design of the clinical research is ethical and that the rights of study participants are protected throughout the study. Some clinical research also requires the approval of a governmental regulatory agency, such as the Food and Drug Administration (FDA) in the United States. Before a new treatment can be marketed as a treatment for a particular condition in the United States, it must be proven safe and effective. The FDA is responsible for ensuring the safety and effectiveness of all drugs, biologics, vaccines, and medical devices.
Clinical Trial Phases
Once a new treatment is approved for first time use in a clinical trial, the treatment will undergo evaluation in a series of clinical trial phases.
A Phase 1 trial is the first step and is designed primarily to evaluate the safety of a drug or treatment in humans. Safe dosage range and side effects are identified. Usually, a Phase 1 trial involves a few healthy volunteers and takes place at only one or two locations, but can include those with the target condition. Investigators use the study results to decide on the best dose and refine procedures for use in further testing.
After completing a Phase 1 trial, investigators will decide whether to seek approval to continue with a Phase 2 trial. A Phase 2 trial will expand the study to a larger group of people with the target condition and compare the results to those of a control group. Those in the control group will receive either the standard treatment or a placebo treatment an inactive substance. The major goal of Phase 2 is to decide if the treatment is effective and to evaluate further its safety. A few hundred people may participate.
If the results of Phase 1 and 2 are promising, a Phase 3 trial may be sought with an even larger and more diverse group of people with the target condition. The goal of this phase is to confirm effectiveness, monitor side effects, and collect information that will allow safe use of the drug or treatment. Investigators compare the results of the new treatment to that of a standard or placebo treatment. Typically, they will perform a randomized controlled trial in which research volunteers are assigned randomly to either a group that receives the new treatment or a control group that receives the standard or placebo treatment. Several hundred to thousands of people may take part.
The final clinical trial phase is a Phase 4 trial. A Phase 4 trial is conducted to evaluate the ongoing long-term safety and effectiveness of a treatment in large and varied populations and to study additional uses of the drug or treatment. This phase usually takes place after the treatment has been made available to doctors and been approved for standard use in treating at least one condition. Hundreds to thousands of people may take part.
Clinical Research Protocols
All clinical trials and studies have a protocol a careful set of rules that set forth how the clinical experiment is to be conducted. The protocol specifies the treatment procedures and doses, the route of administration, the number of participants, the specific inclusion and exclusion criteria that define who may enroll in the study, the duration of the study, and the schedule of the tests or interventions that will measure the results. In trials and studies for SCI, examples of inclusion criteria might be the volunteer's level and type of injury, the time since injury, age, and gender.
An important part of the clinical protocol is the informed consent, which is an ongoingprocess that gives people ample opportunity to learn the important facts about a clinical trial so they can decide whether to begin or continue participation. During this process, an investigator explains what will happen to the research volunteer. Depending on the treatment under investigation, research volunteers may or may not experience a direct benefit because of their participation.
- See more at: http://www.themiamiproject.org/research/what-are-clinical-trials/#sthash.JiQBvKiR.dpuf
8.9. Unite 2 Fight Paralysis
Information on clinical trials from around the world was compiled by a dedicated group of volunteers for the benefit of the spinal cord injury community, and is updated regularly. Unite 2 Fight Paralysis does not imply or infer an endorsement or recommendation of any of the research listed.
For information on additional clinical trials, please visit the clinicaltrials.gov website. There is also a search feature that allows you to search for clinical trials by location.
Questions and comments are welcome and should be directed to: trials@unite2fightparalysis.org
(Feb 2016)
9. Will my insurance reimburse for participating in SCI research?
9.1. Coverage for Experimental Technologies (1995)
In this paper in PDF format the authors argue that had insurers, including Medicare, not paid the costs associated with “unproven” technologies in the past, many of the innovations for which American medicine is lauded might not have come to pass.
9.2. Appeal for Experimental Procedures
This article explains terminology to help you overcome the challenges that can arise when your medical needs are not covered by your health insurance.
9.3. Experimental Cure Tough Choices (1999)
Article from the Seattle Times Extra "Experimental cures mean tough choices for health insurers"
10. Websites to learn about SCI research
10.1. Unite 2 Fight Paralysis
Unite 2 Fight Paralysis exists to unite and empower the international spinal cord injury community to cure paralysis through advocacy, education, and support for research. United 2 Fight Paralysis offers multiple ways to learn about the latest research including podcasts, newsletters, and their annual Working 2 Walk Symposium. Their CureCast podcasts cover a wide range of SCI research approaches.
Contact Information:
Unite 2 Fight Paralysis
528 Hennepin Ave. Suite 606
Minneapolis, MN 55403
Email: unite@u2fp.org
Phone: 1-888-564-2228
10.2. Miami Project to Cure Paralysis
The Miami Project to Cure Paralysis is a premier spinal cord injury research center. The Miami Project's international team of more than 175 scientists, researchers and clinicians take innovative approaches to the challenge of spinal cord injury. Miami Project researchers conducting clinical studies and trials in spinal cord injury, including testing neuroprotective strategies, cellular therapies, and advanced rehabilitation and neuromodulation approaches.
10.3. Christopher & Dana Reeve Foundation
The Reeve Foundation's research platforms are developing and delivering treatments and cures for spinal cord injury.
10.4. CenterWatch - Clinical Trials information
Global source of news, directories, proprietary market research, and analysis for clinical trials professionals and patients.
10.5. Mike Utley Foundation
The mission of the Mike Utley Foundation is to financially support an effective function-restoring treatment for spinal cord injuries, to encourage through education that of adopting a rehabilitative lifestyle for the spinal cord injured and a public awareness of spinal cord injuries.
11. Articles and Videos about SCI research
11.1. Spinal Cord Injury Research and the Hope for Cure: Where are we Today? (2013)
2013 Spinal Cord Injury Wellness Summit
Keynote address:
SCI Research and the Hope for Cure: Where Are We Today?
By Daniel Lammertse, MD
Internationally recognized as an expert in SCI rehabilitation, Dr. Lammertse is a Clinical Professor of Physical Medicine and Rehabilitation at the University of Colorado, Denver, and Medical Director of Research at Craig Hospital, where he is co-director of the Rocky Mountain Regional SCI System. In his talk, he lucidly explains the complex issues involved in SCI recovery, reviews the current state of SCI cure research worldwide, discusses the most promising avenues of investigation, and clarifies potential risks of participating in expensive experimental treatments available outside the U.S. Dr. Lammertse's talk was the keynote address at 2013 SCI Wellness Summit, which took place on May 18, 2013 at the University of Washington Medical Center, Seattle.
11.2. Spinal Cord Injury Research Evidence (SCIRE)
The Spinal Cord Injury Research Evidence (SCIRE) is a synthesis of the research evidence underlying rehabilitation interventions to improve the health of people living with SCI. SCIRE covers a comprehensive set of topics relevant to SCI rehabilitation and community re-integration.
The Spinal Cord Injury Research Evidence (SCIRE) project is a Canadian collaboration between scientists, clinicians and consumers in Vancouver, British Columbia and London, Ontario and their respective health centres (GF Strong Rehab Centre, St. Joseph's Health Care), research institutions (International Collaboration on Repair Discoveries, Lawson Health Research Institute) and universities (University of BC, University of Western Ontario).
11.3. CareCure Forums - Article Abstracts
The Research Forums lists abstracts of the latest scientific articles on brain injury & stroke, neurodegeneration, multiple sclerosis, neuropathic pain, spinal cord injury, stem cells, and tranverse myelitis. Exchange information about Clinical Trials, Equipment & Services, Doctors & Clinics.
11.4. Hope Through Research (NINDS)
This brochure by the National Institute of Neurological Disorders and Stroke (NINDS) has been written to explain what happens to the spinal cord when it is injured, the current treatments for spinal cord injury patients, and the most promising avenues of research currently under investigation.
11.5. Research: Stem Cells
Research: Stem Cells, is a brochure produced by Craig Hospital, a Spinal Cord Injury Model Systems Center. Revised 1/15.
11.6. Stem Cell Therapies
Stem Cell Therapies
Will stem cell therapies be safe and effective for treating spinal cord injuries?
A large number of different cells, including embryonic and adult stem cells, have been transplanted into animals with SCI, and in many cases these procedures have resulted in modest sensorimotor benefits. This review article examines some of the publically available preclinical evidence that some of these cell types improve outcome in animals with SCI. Transplantation of many different types of stem and progenitor cells acutely after SCI enhances spontaneous recovery of function in animals. The common mechanism(s) of this enhanced recovery of function are not well understood, although a range of possibilities are usually cited (including preservation of tissue, remyelination, axon sprouting, glial cell replacement). There is no agreement about the best cell type for transplantation. Transplantation of cells into animals with a long lifespan is important to determine whether or not tumors will eventually form. It will also be important to determine whether long-term survival of cells is required for functional recovery.
Thomas KE, Moon LD.
Br Med Bull. 2011;98:127-42.
How to obtain complete article
You may obtain copies of the complete article through your local medical library or through the University of Washington Health Sciences Library Document Service at 206-543-3441 or http://healthlinks.washington.edu/hsl/docservices/illiad.htm. (There is a fee for this service.)
11.7. Survive, Subsist, Succeed: Spinal Cord Injury
Survive, Subsist, Succeed: Spinal Cord Injury
An 80+ minute video presentation by John D. Steeves, PhD, Professor and Founding Director, ICORD (International Collaboration On Repair Discoveries), and UBC and Vancouver Coastal Health, Vancouver, BC. In this presentation, Dr. Steeves addresses questions about fact vs fiction, near term vs long term in relation to research and provides an overview of the SCI research that has taken us from merely surviving spinal cord injuriesrare before World War IIto thriving with SCI and even the possibility of recovery. He brings over 30 years' experience as a spinal cord researcher to explain and discuss recent experimental treatments for SCI.
This lecture was given on May 20, 2010 at University of Washington Medical Center by the Northwest Regional SCI System, Department of Rehabilitation Medicine, University of Washington.
12. Advocacy Groups
12.1. Cure Paralysis Now
CureAdvocacy is a grassroots organization that believes a cure for paralysis can be achieved in this decade.
12.2. Step Now
The purpose of the site is to explore and develop global plans to aid in the fight for a cure for paralysis.
12.3. Spinal Cord Society
SCS is a large grass roots organization linked by a monthly Newsletter, and thousands of members throughout North America and other countries. Its goal is cure of chronic spinal cord injury paralysis.
12.4. Unite 2 Fight Paralysis
Unite 2 Fight Paralysis is a nonprofit organization dedicated to the education, unification and empowerment of all survivors of paralysis.
13. SCI Statistical sources
13.1. 'One Degree of Separation' paralysis survey 2009
'One Degree of Separation': Paralysis and Spinal Cord Injury in the United States.
13.2. National SCI Statistical Center (At UAB)
The UAB Department of Physical Medicine and Rehabilitation is funded by the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) to operate the National Spinal Cord Injury Statistical Center (NSCISC). NSCISC supports and directs the collection, management and analysis of the world's largest and longest spinal cord injury research database. Organizationally, NSCISC is currently at the hub of a network of 14 NIDILRR-sponsored and 5 subcontract-funded Spinal Cord Injury Model Systems located at major medical centers throughout the United States. In addition to maintaining the national SCI database, NSCISC personnel conduct ongoing, database-oriented research. Many of the findings resulting from these investigative efforts have had significant impact on the delivery and nature of medical rehabilitation services provided to SCI patients.
"Traumatic Spinal Cord Injury Facts and Figures at a Glance, 2023" is a publication of the National Spinal Cord Injury Statistical Center, Birmingham, Alabama. Much additional SCI statistical data and annual reports can be accessed from this page.
The NSCISC Life Expectancy Calculator allows you to perform a more accurate personal life expectancy assessment.
13.3. Annual Disability Statistics Compendium
The Annual Disability Statistics Compendium is a web-based tool that pools disability statistics published by various federal agencies together in one place. When working on legislative and other matters relating to persons with disabilities, the Compendium will make finding and using disability statistics easier.
13.4. Geography of Disability
The NIDILRR-funded Rehabilitation Research and Training Center on Disability in Rural Communities (RTC: Rural) has published findings from the Geography of Disability Project on their website. The objective of the study was to depict the distribution and demographics of people with disabilities living in rural areas and the services available to them. A number of map formats are available.